
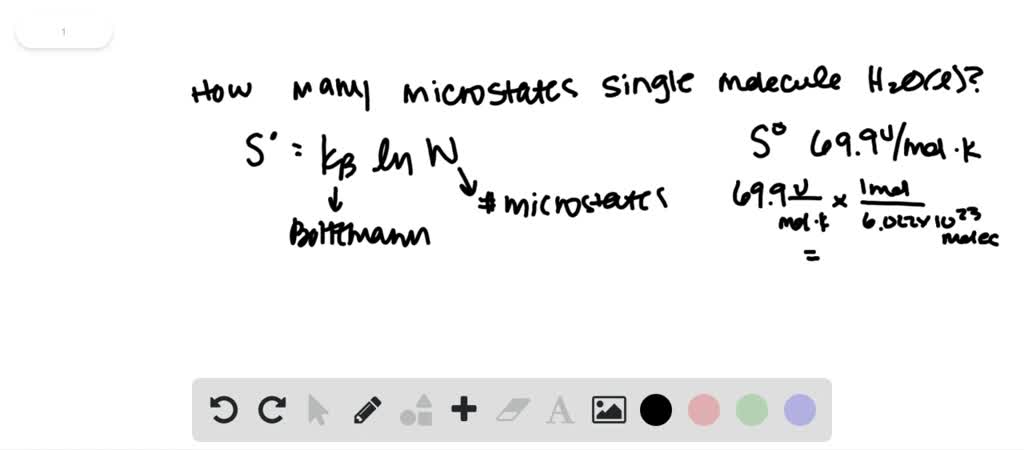
However, you won't see calculations for simple 'entropy' because the concept is fairly useless until you put it in a form that can be used to make c. The capital letter S is used to denote entropy. Do the natural log of to multiply it by eight point for you, For that's here, you're gonna get answer of 5.76 jewels were Covance. Answer (1 of 3): Entropy is a measure of the randomness, chaos, or freedom of movement of particles. 19, we obtain the following expression for the molar. (20) S S v + S t + S r Substituting the vibrational partition function given in Eq. Um, I was going to calculate this answer, and I'm gonna plug it into my calculator real quick. The entropy is given by : (19) S k B l n Q + k B T ( l n Q V) T The molar entropy is a combination of three contributions, including vibrational, translational and rotational entropies. So after all this is said and done, we couldn't finally plug it in.
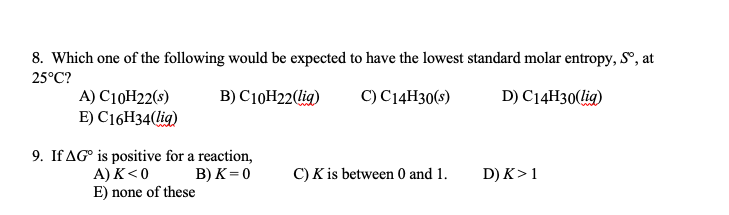
And then, since we're working with Delta s, which is in terms of units, units of jewels, we're going to use our as 8.314 Jules, her mobile times, Calvin instead of 0.8 through 14 and the natural log of the change in volume, which is 2.0 leaders minus 1.0 leaders. Standand Enthalpies of Formation & Standard Entropies of Common Compounds Substance State H f S (kJmol) (JmolK) Ag s 0 42.6 Ag+ aq 105.79 72.7 AgCl s 127.01 96. that maximizes the information entropy of c(M) (Press et al. Just say that there's 1.0 molds of a gas in this system because It just wants us to calculate the temp changing and should be for the system. cient distributions c(s) or molar mass distributions c(M). That's what we're trying to find equals, um, so to start off with and we're not really given a number of moles from this problem, so we can simplify this. We're gonna go ahead and play in what we know. So this is proportional to that original equation. Is it BF v eyes proportional to the amount of micro states? Because as you have a higher about for you, number of micro sees change. So the yes minus three I so final my initial in this portion right here. S/n, is expressed in joules per kelvin per mole (JK'mol') molar entropy is an intensive. It's still too s is equal to, and the number of moles are saying constant We would love in the natural log of instead of the micro States, That's going to be the change in volume. Molar entropy, the entropy divided by the amount of substance, S.

We've got initial volume one leader final volume of two liters and we're going to use the equation similar to Bolton's equation, but has slight variation. So working to calculate entropy change for a different kind of problem.


 0 kommentar(er)
0 kommentar(er)
